
Introduction: Chimeric Antigen Receptor (CAR) T cell therapy targeting CD19 has shifted our treatment approach for relapsed and refractory (r/r) pediatric B cell acute lymphoblastic leukemia (ALL). The landmark ELIANA pediatric trial studying tisagenlecleucel, CD19-specific CAR T cells, demonstrated a complete response (CR) rate of 81% in 75 infused patients and 12 month overall survival (OS) and event-free survival (EFS) rates of 76% and 50% respectively. Cytokine release syndrome (CRS) and neurotoxicity rates of 77% and 40% were respectively reported. In August 2017, the FDA approved tisagenlecleucel for B-cell ALL that is refractory or in second or greater relapse in patients up to age 25. With CAR commercialization, institutions deliver tisagenlecleucel without the regulation of a clinical study and practices relating to CAR delivery and reporting remain heterogeneous. Here, we report real world clinical outcomes using commercially available tisagenlecleucel for pediatric r/r B-ALL.
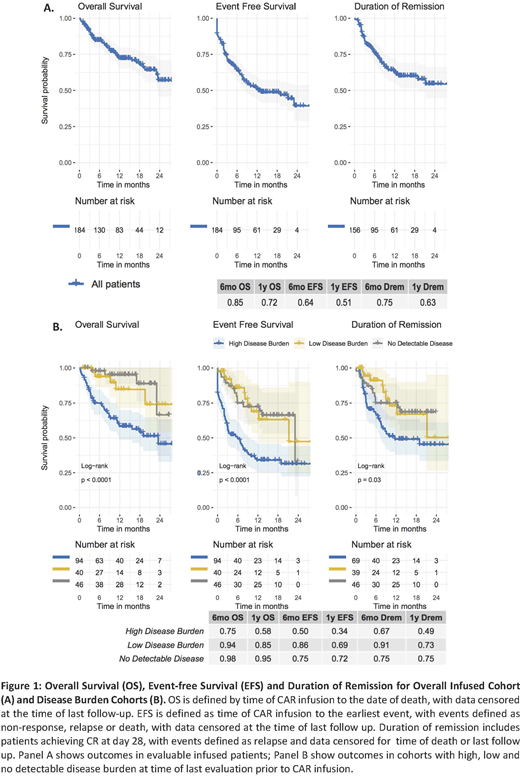
Methods and Results: Retrospective data were collected from PRWCC member institutions (n=15) and included 200 patients. This includes 15 (7.5%) patients not infused due to manufacturing failure (n=6), death from disease progression and/or toxicity (n=7), or physician discretion following disease remission from prior therapy(n=2). The remaining 185 patients (92.5%) were infused with tisagenlecleucel, including 87% (161) receiving standard-of-care CAR T cell products meeting manufacturing release criteria and 13% (24) receiving CD19-CAR T cells manufactured by Novartis and provided on the managed access program (NCT03601442; n=14) or with single-patient IND approval (n=10). At time of CAR T cell infusion, median age was 12 years (range 0-26) with 40% females and 60% males. Median duration of follow-up at time of analysis was 11.2 months (range 0.2-28.8). The CR rate at 1 month follow up was 79% (156/198) on an intent-to-treat basis and 85% (156/184) among evaluable infused patients. Of infused patients achieving morphologic CR with available testing, 97% (148/153) were negative for MRD by flow cytometry. Duration of remission at 6 and 12 months among patients who achieved CR was 75% and 63% respectively, with 35% (55/156) of responders experiencing relapse. At time of relapse, 41% (21/51) of evaluable patients had relapse with CD19- disease and 59% (30/51) had continued CD19 expression. OS and EFS rates were 85% and 64% at 6 months and 72% and 51% at 12 months, respectively. CRS and neurotoxicity of any grade were seen in 60% (111/184) and 22% (39/181) of evaluable patients with ≥ grade 3 CRS and neurotoxicity rates of 19% (35/184) and 7% (12/181) respectively. One grade 5 CRS and 1 grade 5 neurotoxicity (intracranial hemorrhage) were reported. Post infusion toxicity management included tocilizumab in 26% (47/184) and systemic steroids in 14% (25/184) of patients. Among 181 infused patients with documented disease burden, 51% (95) had high burden (HB) disease , as defined by >5% bone marrow lymphoblasts, peripheral blood lymphoblasts, CNS3 status or non-CNS extramedullary (EM) site of disease; 22% (40) had low burden (LB) disease, defined by detectable disease not meeting the HB criteria; and 25% (46) had no detectable disease (NDD) at time of last evaluation prior to CAR infusion. The morphologic CR rate was lower at day 28 in HB vs. LB and NDD (74% vs. 98% and 96%) and the OS and EFS were lower among patients with HB at 6 mo [OS; 75% (HB), 94%(LB), 98% (NDD), EFS; 50% (HB), 86% (LB), 75%(NDD), p<0.0001] and 12 mo [OS; 58% (HB), 85% (LB), 95% (NDD), EFS; 34% (HB), 69%(LB), 72%(NDD), p<0.0001]. Multivariate analysis will be presented at the meeting.
Conclusions: This retrospective, multi-institutional analysis describes real world outcomes using tisagenlecleucel to treat pediatric r/r B-ALL. Early responses at 1 month and OS and EFS at 6 and 12 months are comparable to reported ELIANA trial outcomes. Safety is demonstrated in this cohort with lower rates or CRS and neurotoxicity, likely related to a lower disease burden cohort. Continued relapse and decrease in OS without evident plateau is seen following 6 months post-infusion warranting expanded follow up. Comparative analysis of outcomes in patient cohorts with varying disease burden demonstrate decreased CR, EFS and OS in patients with high disease burden as compared to patients with lower disease burden or no detectable disease at last evaluation prior to CAR infusion.
Phillips:Novartis: Membership on an entity's Board of Directors or advisory committees. Stefanski:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Margossian:Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Verneris:Fate Therapeutics: Consultancy, Current equity holder in publicly-traded company; Novartis: Membership on an entity's Board of Directors or advisory committees; Bmogen: Consultancy, Current equity holder in publicly-traded company; Uptodate: Consultancy. Myers:Novartis: Consultancy, Honoraria, Other: ELIANA trial Steering Committee, Speakers Bureau. Brown:Jazz: Honoraria; Servier: Honoraria; Janssen: Consultancy; Novartis: Consultancy. Qayed:Novartis: Consultancy; Mesoblast: Consultancy. Hermiston:Novartis: Membership on an entity's Board of Directors or advisory committees; Sobi: Membership on an entity's Board of Directors or advisory committees. Satwani:Takeda: Consultancy; Mesoblast: Consultancy. Curran:Novartis: Consultancy, Research Funding; Mesoblast: Consultancy; Celgene: Research Funding. Mackall:Lyell Immunopharma: Consultancy, Current equity holder in private company; Nektar Therapeutics: Consultancy; NeoImmune Tech: Consultancy; Apricity Health: Consultancy, Current equity holder in private company; BMS: Consultancy; Allogene: Current equity holder in publicly-traded company. Laetsch:Cellectis: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Research Funding; Bayer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal